Atomic Structure and the Periodic Table
What we are learning:
Elements, Compounds and Mixtures:

Elements are found on the periodic table and there are approximately 100 of them. They have specific symbols that match to their names. These symbols are used to represent them as both elements and in parts of a compound. A compound consists of two or more elements chemically bonded. There is a common misconception that O2 is a compound as there are two atoms chemically combined, however, these are identical so O2 is still an element. Mixtures contain different elements and/or compounds that are not chemically bonded and do not react with each other. Air is a great example of a mixture as there are elements: N2, O2 and Ar and the compound CO2 all in the same space.
Structure of Atoms:

The nucleus of an atom contains the protons and neutrons (only one isotope of Hydrogen has no neutrons). The nucleus is tiny compared to the rest of the atom but it contains most of the mass. Protons have a +1 charge and neutrons have no charge. As atoms have zero overall charge, these positive protons must be balanced out by an equal number of electrons that have -1 charge. Electrons orbit the nucleus in shells and have almost no mass (1/2000 that of a proton or a neutron). The number of protons in an atom is equal to the atomic number for an element on the periodic table. The atomic mass shows the number of particles in the nucleus (the nucleons). To work out how many neutrons there are, subtract the atomic number from the atomic mass. Isotopes of an element must have the same number of protons (or it would be a different element) but they have differing numbers of neutrons.
Atoms generally have a radius of 1 x 10-10 m and the nucleus will only have a radius of 1 x 10-14 m.
Electronic structures of atoms and ions:

The electrons orbit the nucleus in different shells or energy levels. The first shell can only fit 2 electrons, the second can fit 8, the third can fit 8 and as we are only interested in the first 20 elements (Hydrogen to Calcium), we only need space for 2 in the 4th shell. This gives Calcium the electron structure of 2:8:8:2.
You will see the patterns that show that the group number is also the number of electrons in the outer shell (except group 0 which has 0 electrons in the next one). This also gives rise to the charges on ions as metals lose their outer electrons to gain a full outer shell, becoming positive ions and non-metals accept these electrons to fill their outer shells and become negative ions.
The Model of the Atom:

We see images that represent the structure of the atom but really, we cannot see them how we see other things around us today. Our understanding of the atom comes from results and interpretations of experiments. Starting with Dalton's Solid Sphere, this was improved when JJ Thompson proposed the Plum Pudding model. Later, Rutherford's team found that the atom was mainly empty space with a tiny positive nucleus in the gold foil experiment, this gave rise to the Nuclear model. The next big change was when Bohr quantised the electron energy levels as electrons cannot change their distance from the nucleus without gaining or releasing energy. This is the Planetary model which is the most useful to us chemists today.
This is a great summary of the history of the model of the atom
Experiments showed the presence of the proton which is given a whole number and, in atoms, is the same as the number of electrons. The work of James Chadwick identified the third sub-atomic particle that we know as the neutron that has no charge and is found in the nucleus.
Separation Techniques:

For mixtures, you need to understand ways of separating them. Distillation will separate 2 liquids such as alcohol in water. Sieving will separate large items from a finer solid or liquid. To get fine solids from a liquid, use filtration. A magnet will remove magnetic metals from liquids or solids. Finally, to separate the pigments from each other in an ink, we use chromatography. You will meet this final technique again in the future when we look at chemical analysis.
The Development of the Periodic Table:

Today, the periodic table that we are used to has the elements in order by their proton number and grouped by similar properties. We have also seen that these groups also have the same number of electrons in their outer shell. Before the discovery of protons, elements were placed in order of their atomic weight which did not work (example of Iodine and Tellurium). Many people tried to order the elements but as they did not know about protons and as many elements had not been discovered, they did not work well. Mendeleev's system came up with a compromise that that used weights but he did two important things: He left gaps for undiscovered elements and he prioritised properties over weights (he put Iodine in group 7 as it behaved like chlorine & bromine even though the weight would have put it in group 6). Elements that were later discovered fit perfectly into the gaps that he left. Later discovery of the proton by Henry Moseley proved that this was the correct way of positioning the elements.
Group 1 - The Alkali Metals:
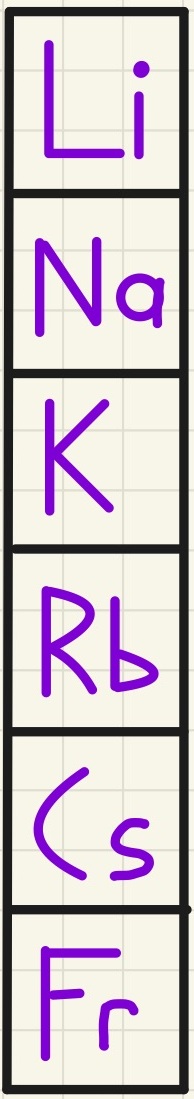
You will get a chance to see how lithium, sodium and potassium behave when they are placed in water. All of these react to give off hydrogen gas and leave an alkali solution, hence their name of the Alkali Metals. The formula is:
2Na(s) + 2H2O(l)→ 2NaOH(aq) + H2 (g)
These all have similar chemical properties as they have 1 electron in their outer shell, however, as you go down the group, they become more reactive. The increase in reactivity is because as the atoms get bigger (further down the group, they have more electrons so more shells to fit them into), the outer electron is further away from the positive nucleus that attracts it. The electrons in between "shield" this attraction so there is more shielding in bigger atoms so the outer electron is more easily lost.
Group 7 - The Halogens:
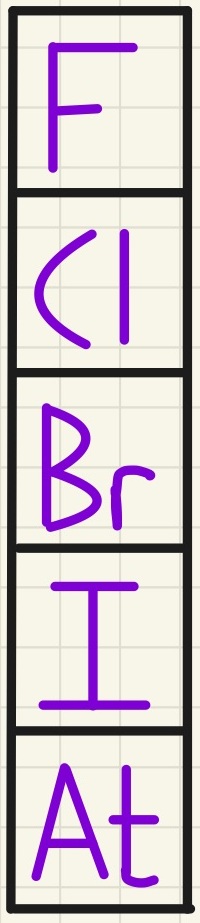
The halogens are all non-metals and all have 7 electrons in their outer shell. This causes them to have similar chemical properties as they each need to gain one more electron. You will learn later how they bond with each other in a covalent manner, and form diatomic molecules such as F2, Cl2, Br2 and I2. We do not concern ourselves with the chemistry of astatine as there is so little on the planet (approximately 1g in the entire crust at any time), but we know that it will follow these patterns.
Reactivity increases as you go up the group (opposite to group 1). This is because, unlike with metals, these atoms wat to gain an electron to fill their outer shell. As it is the nucleus that can attract other electrons, the small atoms have a greater pull on the electrons nearby. The melting points and boiling points increase as you go down the group.
Finally, note that more reactive halogens can displace less reactive ones from their compounds. Chlorine will displace iodine from sodium iodide but it cannot displace fluorine.
2NaI(aq) + Cl2 (aq)→ 2NaCl(aq) + I2 (aq)
Whereas
2NaCl(aq) + Br2 (aq)→ NO REACTION
Group 0 - The Noble Gases:
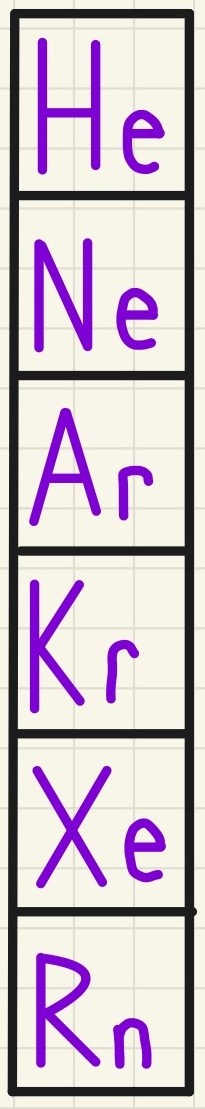
Group 0, sometimes called Group 8 houses the Noble Gases. These are very unreactive elements that all have full outer shells of electrons so they do not need to gain or lose any so they do not react. As Helium has 2 electrons in its full outer shell and the others have 8 in their full outer shell, there is the confusion as to the name of the group, this is why they are called Group 0. The boiling point increases as you go down the group as does the density of the gas. These are used for their unreactive properties such as Argon in light bulbs and Helium in balloons - this is a combination of very low density and unreactive behaviour making it an excellent alternative for airships to avoid disasters such as with the Hindenburg.
Key words/terms for this topic
Abundance Alkali Metals Aqueous Atom Atomic number Chemical properties Chromatography Compound Condenser Crystallisation Displacement Distillation Electron Electron Shell Element Energy level Filtration Formula Group Groups Halogens Inert Ion Isotope Mendeleev Neutron Noble Gases Nuclear Nucleon Nucleus Orbit Organised Period Periodic Table Periods Pigment Plumb Pudding Model Product Proton Reactant Reactive Reactivity Relative atomic mass Relative charge Relative mass Shell Solute Solution Solvent Unreactive
Curriculum Health Check:
Q: How can you calculate the number of neutrons in an atom?
A: Atomic number
B: Atomic mass - atomic number
C: Atomic number - atomic mass
D: Atomic mass
What you need to know
Everything is made from the approximately 100 types of atoms on the periodic table. Elements are made from one type of atom, compounds are atoms of different elements chemically combined (bonded) and mixtures are two or more elements or compounds not chemically combined.

You need to select, describe and explain techniques of separation.

You need to know about the first 20 elements only (Hydrogen to Calcium), their patterns and reactions.

The model of the atom, what it is now, how it was first described and steps along the way as the model was improved due to experimental work (links with Physics).

Particles that make up an atom, their sizes, where they are found, their charges and the structure of electron shells. You should also know the approximate sizes of atoms: radius = 0.1 nm (or 1 x 10-10 m), the radius of the nucleus is 1/10 000 of the size, (approximately 1 x 10-14 m).

Relative atomic mass, describe the symbols on the periodic table and explain the mass number and atomic number with links to isotopes.

You need to know how the periodic table was developed from the initial handful of elements known to what we have today. Describe the input that certain scientists had.

Finally, you need to know, in detail, the reactions and properties of groups 1, 7 and 0. Patterns in reactivity and size will need to be explained.

This page was updated on: 15th February 2024

