Particle Model of Matter
What we are learning:
Density:

Density is a very important concept that is used wrongly in everyday life. I hear people say that "aluminium is light" or "lead is heavy". In fact, 5 kg of lead has the same mass as 5 kg of aluminium. Putting it like that makes it obvious but we must be precise with our language. We use aluminium to make aircraft parts because it is low density.
Density is a measure of how much mass a certain volume of a substance has. In physics, we quote the density in kg/m3. To calculate density, we use this formula:
Density (kg/m3) = mass (kg)/ volume (m3)
Ρ = m/V
One of the required practicals is to find the density of both regular shapes (measuring the mass on a balance and measuring and calculating the volume using a ruler or micrometer then finding the mass on a balance), and finding the density of an irregular shaped object (mass from a balance then find the volume using a displacement/eureka can).
States of matter:
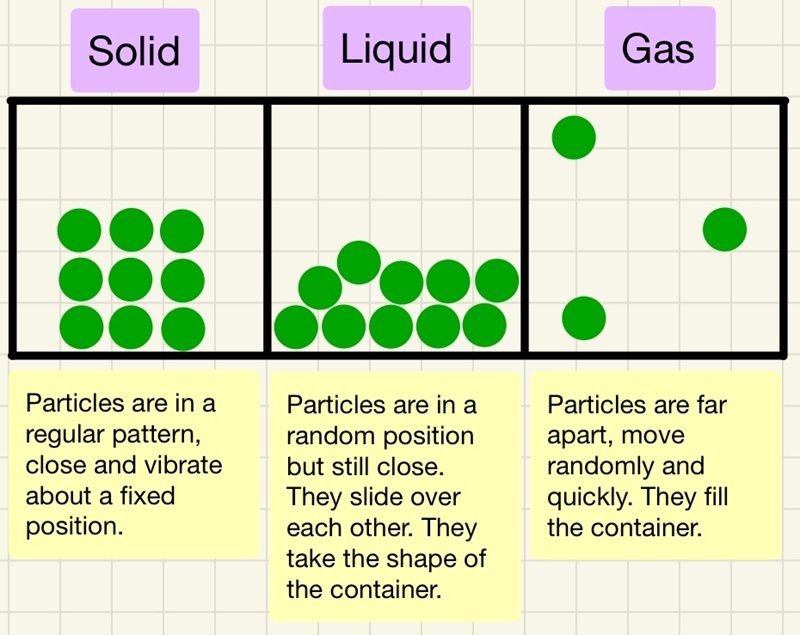
We use models to show what the particles look like in the different states.
Solid: particles are touching and are in a regular arrangement. The shape of the solid is not determined by the shape of the container.
Liquid: particles are mostly touching but are sliding over each other and not in any regular arrangement. These particles flow to the sides of the container, the container determines the shape.
Gas: particles are not touching, they are some distance away from each other and their position is completely random.
This is a great model to explain what is happening, however, there are limitations of the model. The model does not show any of the forces between the particles and particles are not little solid spheres. Now that the model shows how tightly packed the atoms are, the numbers of particles in a certain volume are almost identical for solids and liquids, this gives them a very similar density. Gases have very few particles in the same volume so are lower density. This is why when a bubble of steam forms at the bottom of your kettle on the heating element, it quickly rises to the top. The particles in all 3 states have a reduction in their density when they warm up, this is the cause of convection, the warmer fluids expand, reduce density so rise up until they cool and contract.
The main anomaly is water. Ice has a lower density than water because of some very complicated chemistry. For us, it is the reason that ice floats, interesting for us, not so interesting for the passengers of RMS Titanic.
Make sure that you know: Melting/Solidifying and Boiling/Condensing. Also Sublimation is going from a solid straight to a gas!
Internal energy - heat & temperature:
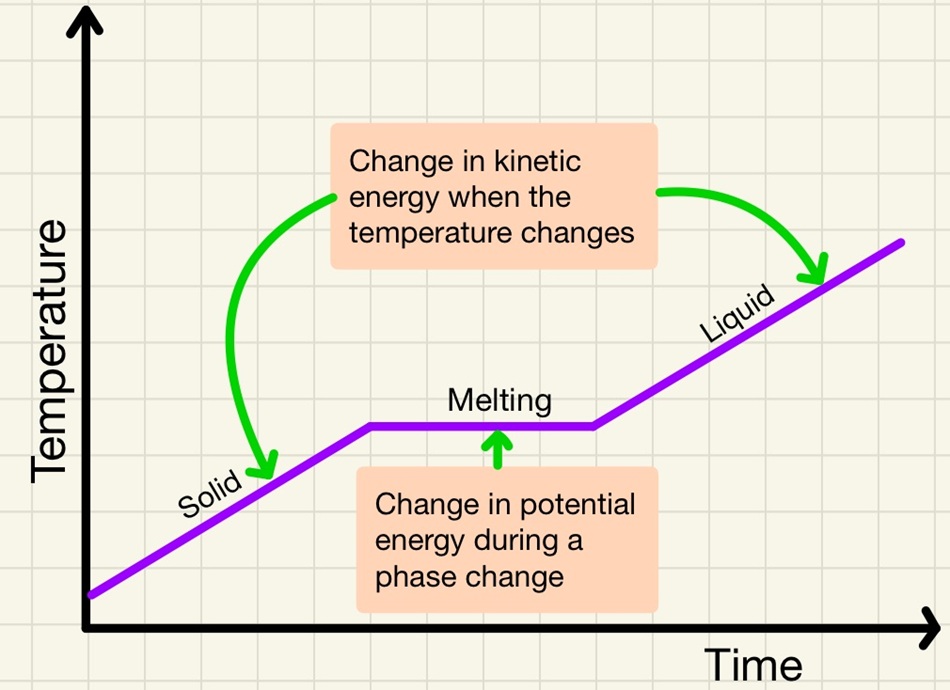
The internal energy in a system is simply the energy stored in the particles. This comes in two parts, firstly there is the kinetic energy. Remember that unless the particles are at absolute zero (0K or -273°C) there will be some vibration. The hotter the substance gets, the more the particles will vibrate. The second part it the potential energy. This is just like elastic potential in that as you heat the particles and they move further apart, they gain potential energy just like pulling either end of an elastic band further apart, the elastic potential increases. With particles, as they move from a liquid to a gas and they move further apart, the potential energy increases, changes in state cause a change in potential energy.
In summary:
• Increase in temperature = increase in kinetic energy.
• Melting and boiling = increase in potential energy.
The last thing to ensure is that we know the difference between heat and temperature. By adding heat, we increase the internal energy of a system. This extra heat will either cause a change of state or an increase of temperature.
Added note, a student became confused, when heating up an ice cube from -5°C to 5°C, what are the changes in internal energy? When warming the ice to 0°C;, you are increasing the kinetic energy. When melting the ice, you are increasing the potential energy (note that the ice cube does nt get any warmer until it is a liquid) and finally, as the now liquid water moves from 0°C to 5°C, the kinetic energy is increasing.
Total internal energy = Potential energy + Kinetic energy
Specific heat capacity:

If you are worried that you are experiencing Déjà vu, fear not, we have already learned about specific heat capacity in the energy topic a few topics ago. I have already covered all of the information in that part but it is worth revisiting it now and applying what we now know about internal energy, particularly the kinetic component of it.
Specific latent heat:

This is a fascinating topic that we can see in real life every winter. Scientist Joseph Black observed that the temperature of a block of ice will keep rising until it reaches the melting point, although it is still absorbing heat energy, it stays at 0°C until it is completely melted then the temperature will begin to rise again. This is because of latent heat. When a substance warms, it is absorbing heat energy and it is all transferred to the kinetic store of the internal energy. When it reaches a phase change, it will continue to absorb heat energy but this is transferred to the potential store as bonds/attractions are overcome. During the phase change, there is no increase in kinetic energy so there is no increase in measurable temperature.
The specific latent heat is the amount of energy needed to change the state of 1 kg of a substance without changing the temperature. We use this formula to calculate it:
Energy to change state (J) = mass (kg) x specific latent heat (J/kg)
E = m L
Note that if you are asked how much energy is needed to heat 2 kg of water from -15°C to 10°C, you need 3 different calculations and add up the total energy:
• E = m c ΔΘ for ice -15°C to 0°C
• E = m L for melting the ice (specific latent heat of fusion)
• E = m c ΔΘ for ice 0°C to 10°C
Additionally, there is the specific latent heat of vaporisation for boiling (liquid to gas). Fusion is less than vaporisation as the increase in potential energy is much higher when going from a liquid to a gas as the particles are pulled much further apart.
Particles and pressure:

The gas particles whizzing around you now are moving randomly (Brownian motion). As the gas gets hotter, the particles have more kinetic energy so the average velocity is higher. These particles are not just hitting you, they are hitting the walls around you and their combined force causes pressure. It may be small but it is easier to see of you have half of a bottle of fizzy drink, screw the lid on tight and squeeze it, the plastic walls give a little. Now shake the bottle and you will feel it is much harder to press in the sides, this is because CO2 molecules have come out of solution and are whizzing around the layer above your drink and colliding with the sides, this is the pressure. If this bottle got hotter, then the particles have more kinetic energy, have more velocity, exert a greater force and generate a greater pressure.
If a pressurised canister like a deodorant can or gas bottle were left in a fire, the pressure would continually increase until the pressure caused an explosion. Most canisters have a pressure relief valve that lets some gas escape at very high pressures to avoid this catastrophe.
Pressure is force/area, Pressure (N/m2 or Pa) = force (N) / area (m2). These tiny particles exert a tiny force but over a whole square metre, there can be a lot.
When you start to learn to drive, check your tyre pressure when the tyres are cold, you now know why this is the case because hot tyres (after the friction of a long drive) will contain hotter air particles...
Key words/terms for this topic
Boil Condense Density Displacement Internal Energy Latent Heat Matter Melt Potential Energy Solidify Sublimation
Curriculum Health Check:
Q: In a phase change graph, what is the flat line between warming liquid and warming gas signify?
A: The change in kinetic energy while the substance melted
B: The change in internal energy while the substance boiled
C: The change in internal energy while the substance melted
D: The change in kinetic energy while the substance boiled
What you need to know
Density is the mass of an object divided by its volume. The volume can be calculated or for an irregular shape, measured using a displacement can or a measuring cylinder.

List all of the potential changes of state and the names of the process of changing between them (boiling, freezing etc.) Internal energy is a measure of the kinetic energy of the particles within a substance, when it is heated, the internal energy of the substance increases until it changes state.

Measuring the change in energy is done by referring to the specific heat capacity:
ΔE = m c ΔT

Change in energy = mass x specific heat capacity x change in temperature.

Energy is needed to "rearrange" the particles in a substance when it changes state, this energy is called the Specific Latent Heat. When changing state, the temperature does not change. E=mL

Energy = mass x specific latent heat.

Finally, describe the random motion of particles in a gas. By changing the temperature at a constant volume will increase the pressure. You must be able to link this relationship and describe how one thing causes the other.

Required practical 17: Measure the density of an object with an irregular shape. Use appropriate equipment and measurements.

This page was updated on: 22nd August 2025

